Polymers, also referenced as flocculants, are regularly used in water treatment to aid solid-liquid separation. They increase the speed and efficiency of solid-liquid separation and help dewater sludge.
When using polymers, it is helpful to manage variables in and outside of your control to increase efficiency. This post highlights how to manage these variables to treat wastewater with polymer effectively. If you’re unfamiliar with polymers, read our post about polymer water treatment first.
Variables in Your Control
- Water and wastewater testing to determine the most efficient chemical chemistry
- Polymer solution strength, consistency, aging, and dosing
Variables Outside of Your Control
- Dirty water (slurry) flowrate changes
- Size of sediment in the slurry
- Characteristics of the sediment in the slurry
These variables are present, whether you use settling ponds, thickeners, DAF clarifiers, centrifuges, or other separating devices. Managing them will increase efficiency and cut costs associated with chemical use and labor.
Managing Variables You Can Control
1. Jar Testing: Determining the Most Efficient Chemical for Your Wastewater
Jar testing isolates the top-performing chemistry for a given water sample through chemical testing trial and error. Coagulants and polymer flocculants are added to a water sample in a controlled setting and measured by parts per million (ppm). Isolating the best chemical ratio and combination can be exhaustive, but it’s the best path to efficient onsite performance.
It is important to keep an open, analytical mind and not enter it with a bias based on past observations or pre-determined outcomes when performing these tests. Using fresh chemical standards prepared to the same solution strength with the same age time is essential. Combining two chemistries often yields the best results, so testing how various combinations interact with the sample is important.
You want to work with a company experienced with different wastewater streams. Understanding the chemistry that works and how much to add to your water stream is necessary for on-site performance. Most companies should provide these details with written results and images or videos of your jar test results.
2. Controlled Administration: Polymer Preparation and Dosing Systems
Next is to use polymer make-down systems that will ensure consistent preparation and dosing of the prescribed chemistry. When using polymer flocculants, you must consider solution strength, solution consistency, solution age, and solution dosing.
These variables are important whether you’re using dry or liquid polymer flocculants. Dry polymer preparation is more complicated and requires more complex machines. In contrast, solution consistency and solution age are easier to control with liquid polymers.

2.1. Solution Strength
Solution strength most often fluctuates due to variations in the incoming dilution water flow. If your dry polymer metering mechanism does not monitor dilution water flowrates, you’ll have inconsistent solution strength. For example, the solution strength will be too high if the dilution water flowrate slows down, but the dry polymer metering does not.
Efficient dry polymer preparation systems use communicating components to keep the dilution water to dry polymer ratio consistent. One example would be a dilution water flow meter connected to the dry polymer metering mechanism. If the dilution water flowrate increases, the flow meter informs the polymer metering device to increase the polymer added.
2.2. Solution Consistency
Solution consistency refers to the individual polymer particle hydration and the distribution and dilution of that particle in the polymer solution. Polymer flocculant, especially non-ionic flocculant, tends to stick together and create “fish-eyes” or partially hydrated flocculant globs. It’s important to keep these clusters from forming by hydrating the flocculant grains as individually as possible.
Further, adding additional dilution and mixing energy helps keep “fish-eyes” from forming after the initial hydration. Mechanical mixing may also be employed, but it is important to remember that flocculants are sheer sensitive. So, too much agitation can cause the long flocculant chains to break into smaller inefficient chains.
2.3. Solution Aging
Another key to proper flocculant preparation is aging time. It takes a minimum of forty-five minutes for the flocculant chain to uncoil and activate. Plus, our internal tests show that aging an additional fifteen to twenty minutes, for a total of sixty to sixty-five minutes, increases flocculant efficiency.
Different polymer make-down systems achieve this aging process in different ways. Some employ a two-tank system, consisting of a “mix” tank and a “day” tank. The first tank receives the hydrated flocculant solution, adds additional water, and activates it with a mechanical mixer. Then after mixing for a prescribed period and once the aging is complete, the solution is transferred to the day tank. It is from the day tank that the flocculant solution is pumped to the process point.
Other polymer preparation systems will use a single tank with multiple chambers, baffles, and weirs. Generally, single tank systems hydrate and dilute the polymer in chamber one, mechanically mix in chamber two and pump from chamber three for discharge. The third draw-down chamber uses sensors to initiate preparation and control aging time. Single tank systems offer a compact single skid option.
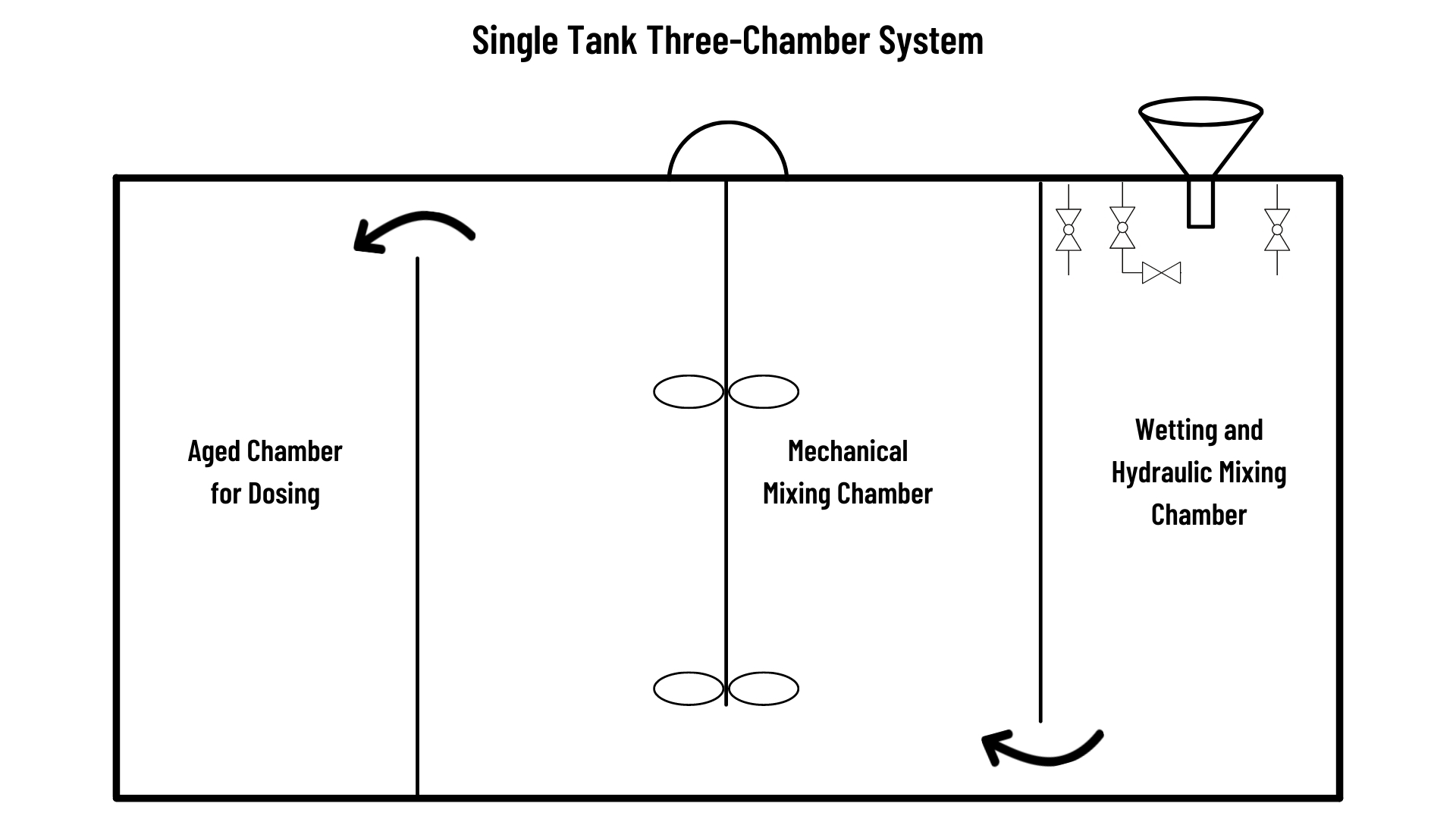
2.4 Polymer Solution Dosing
Polymer solution dosing, also referenced as metering, is the final key to proper flocculant administration. As was touched on earlier, the flocculant chains are highly sheer sensitive. Therefore, it’s important not to break them apart as you dose them into the process stream.
Dosing a flocculant solution without breaking the flocculant chains is achieved by employing a positive displacement pump of some variation. The most commonly used positive displacement pumps are progressing cavity pumps, but gear, rotary lobe, peristaltic, and piston pumps also work.
Coagulant Dosing
As noted earlier, coagulants help separate solids from a liquid by neutralizing the solids electric charge. However, administering coagulant is typically a less complicated process.
While the flocculants cannot withstand a high sheer environment, many coagulants benefit from it. Running coagulants through a high sheer environment such as a centrifugal pump can increase their efficiency. Again, this is not a practice that one would want to employ with most flocculants.
Another differentiating quality between coagulants and flocculants is that coagulants typically do not require much, if any, aging. Coagulants are often administered into process streams with a diaphragm pump as a “neat solution,” meaning it is not diluted with water, aged, or mixed.
Finding the top-performing coagulant can take time when jar testing and is an important step. However, administering coagulants generally does not require complex machines like polymer flocculants.
Variables Outside of Your Control
You’ll still run into variables outside of our control when administering polymer, but they’re manageable. First, we’ll highlight these variables, and then we’ll discuss how to work with them.
Variables outside of your control typically come from variations in the dirty water (slurry) being treated. The most common variables found in slurry streams are changes in slurry flowrates and the amount of solids present, the size of the solids, and the characteristics of those solids.
1. Flowrates & Total Suspended Solids (TSS)
When administering chemicals into a water or wastewater stream, it is important to maintain a chemical to TSS ratio. As noted, a proper jar test measures the ppm so that the amount of chemical administered can scale up or down to the TSS. Proper dosing increases the liquid-solid separation and dewatering efficiency.
However, the ratio determined during jar testing will change if slurry flowrates and percent solids in the slurry change. For instance, a reduction in the solids can cause an abundance of chemical additives which can be costly and detrimental to the system’s stability. Or an abundance of solids can cause the chemical dosing to fail and return dirty water to your process.
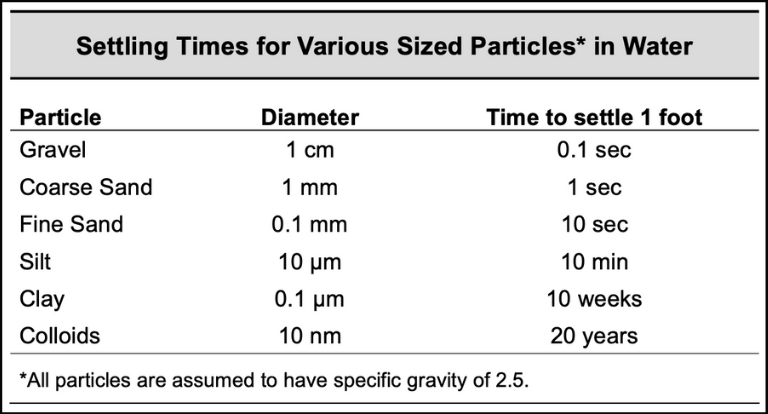
2. Solids Size
A change in the particle size can dramatically alter the effectiveness of the chemistry. Larger particles have a natural propensity to fall out of liquid suspension without the aid of flocculants or coagulants. Conversely, smaller particles have difficulty settling on their own. Therefore, a reduction in the presence of larger particles may seem like a signal for additional chemicals.
However, there is an added element to consider because even a small amount of larger particles can act as ballast. This ballast increases the settling velocity of the “floccule” because of the higher weight it provides. So, going from a small presence of larger particles to a complete absence can impact the sedimentation process and vice versa.
3. Solids Characteristics
Lastly, if the characteristics of the solids in your process stream change, they may not interact with the chemistry as they had previously. Most users respond by adding more chemicals, negatively impacting the process and throwing the system out of balance.
The best approach to changes in solids make-up is to perform another jar test. If the material has changed and the chemical interaction is inefficient, isolating new chemistry is necessary.
Managing Variables Outside of Your Control
Flocculation Monitoring & Automatic Chemical Dosing
The variables noted above can be managed with a flocculation monitoring and chemical dosing adjustment system. Flowrate, percent solids, and to a certain extent, particle size and solids make-up can be adjusted for in real-time by an automatic monitoring and dosing instrument.
One such technology is similar to a turbidity sensor. This technology shoots an infra-red light beam across a sample of slurry and records the amount of light lost to indicate the performance of the chemistry.
Good flocculation chemistry brings the suspended solids together into macro-flocs or clusters of solids. When this occurs, it leaves vacancies between the clusters that the light can pass through with little loss. When the chemistry is inefficient, macro-flocs don’t form properly, the solids absorb more light, and the instrument receives less light.
In both scenarios, the auto-monitoring and dosing system will adjust the chemical dosing rate accordingly. For instance, if a slurry’s percent solids increase, the system will increase the chemical solution dosing rate accordingly. Vice versa if the percent solids decrease.

In conclusion, it is in your best interest to identify and manage as many variables as possible to ensure efficient chemical performance. Proper chemical selection and carefully selected equipment can minimize upset conditions, reduce chemical consumption, and limit operator attention requirements. Uptime is essential to the success of any process, and you can increase production by managing these variables.


