Polymers are everywhere. Nearly every product we touch contains polymers, from a wood desk to plastic water bottles. They’re likely in whatever you’re using to read this post.
However, they don’t just make products. Specific, water-soluble polymers are widely used in wastewater treatment to remove suspended solids and/or contaminants from the water. They’re used regularly in municipal, industrial, and stormwater treatment systems, but many consumers aren’t aware of their importance.
Polymers are nothing short of incredible. In many cases, one can watch polymer solutions settle solids out of liquid suspension in real-time. The liquid/solid separation that once took days or even months when left to gravity alone can typically be achieved in minutes or seconds with properly prepared, activated, and applied polymer.
Although awareness of polymers’ benefits is growing, many industries and individuals are unaware of how polymers can help clarify their wastewater, saving them time, money, and energy. This post will discuss what polymers are and how they can help companies manage their wastewater while ensuring compliance with regulations.
What Are Polymers?
Polymers are a chain of molecules made up of smaller molecules called monomers. Monomers come together to form a chain, and the chain of monomers is called a polymer. There can be millions of monomer molecules in one polymer, and the more monomers there are, the longer the polymer chain.
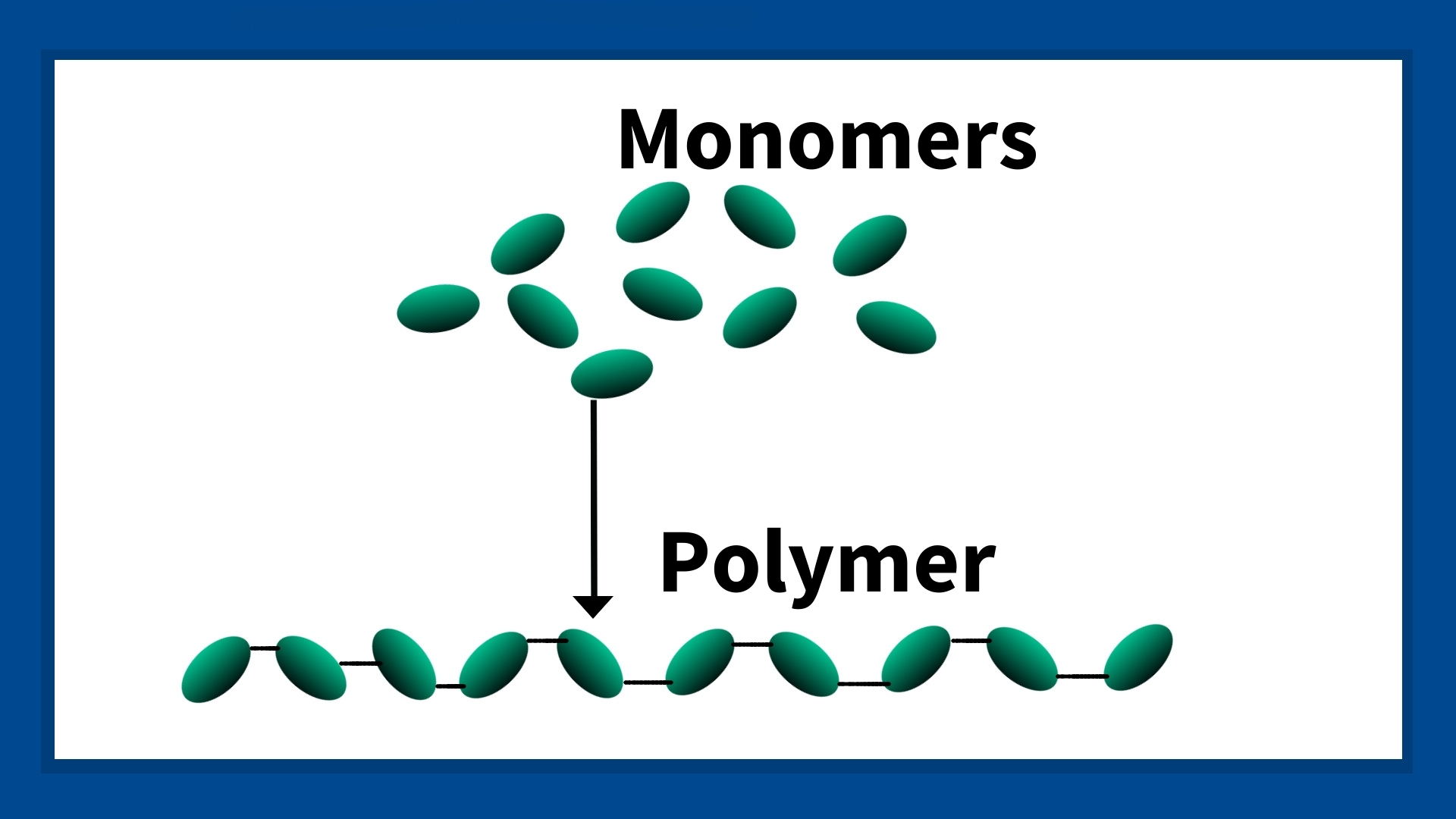
Now, different monomers will create either anionic or cationic polymers. Both polymer types settle liquid-suspended solids, but they’re used in different applications.
Anionic polymers have a negative charge and are commonly used in applications with inorganic solids such as clays and silts. We expect to see anionic polymers used for industrial applications like sand and gravel production, drilling, mining, and other mineral-based liquid-solid separation.
Cationic polymers have a positive charge and are often used to settle organic solids such as animal waste or vegetation. Cationic polymers are used in dredging, municipal wastewater treatment plants, food processing, agricultural and dairy applications.
Dry vs. Liquid Polymer
Wastewater treatment polymers usually come in either a dry granular form or a liquid form.
The liquified polymers are known as emulsions and contain surfactants and emulsifying agents. They are roughly 1/3 equal parts, and the agents are required to keep the flocculant portion in a readily available condition.
Dry and liquid polymers have different concentrations that determine their efficiency and benefit to an operation.
For instance, dry polymers generally have +90% active chemistry vs. 30% active chemistry in emulsion polymers. However, upfront equipment costs for dry polymer systems are usually higher than emulsion polymer systems.
When determining which type to use, buyers need to consider the dosing equipment’s capital cost, the amount of polymer an application requires, whether performance gaps exist, operator attention necessary, and the shelf life. More information about the pros and cons can be found in this dry and emulsion polymer post.

Polymers in Wastewater Treatment
Polymers separate solids from liquids through a process called flocculation. Due to the name of the process, you’ll hear these specific water-soluble polymers referenced as flocculants or polymer flocculants.
The polymers’ ability to flocculate solids is central to their role in water treatment. They can be effective on their own and very impactful when combined with a coagulant.
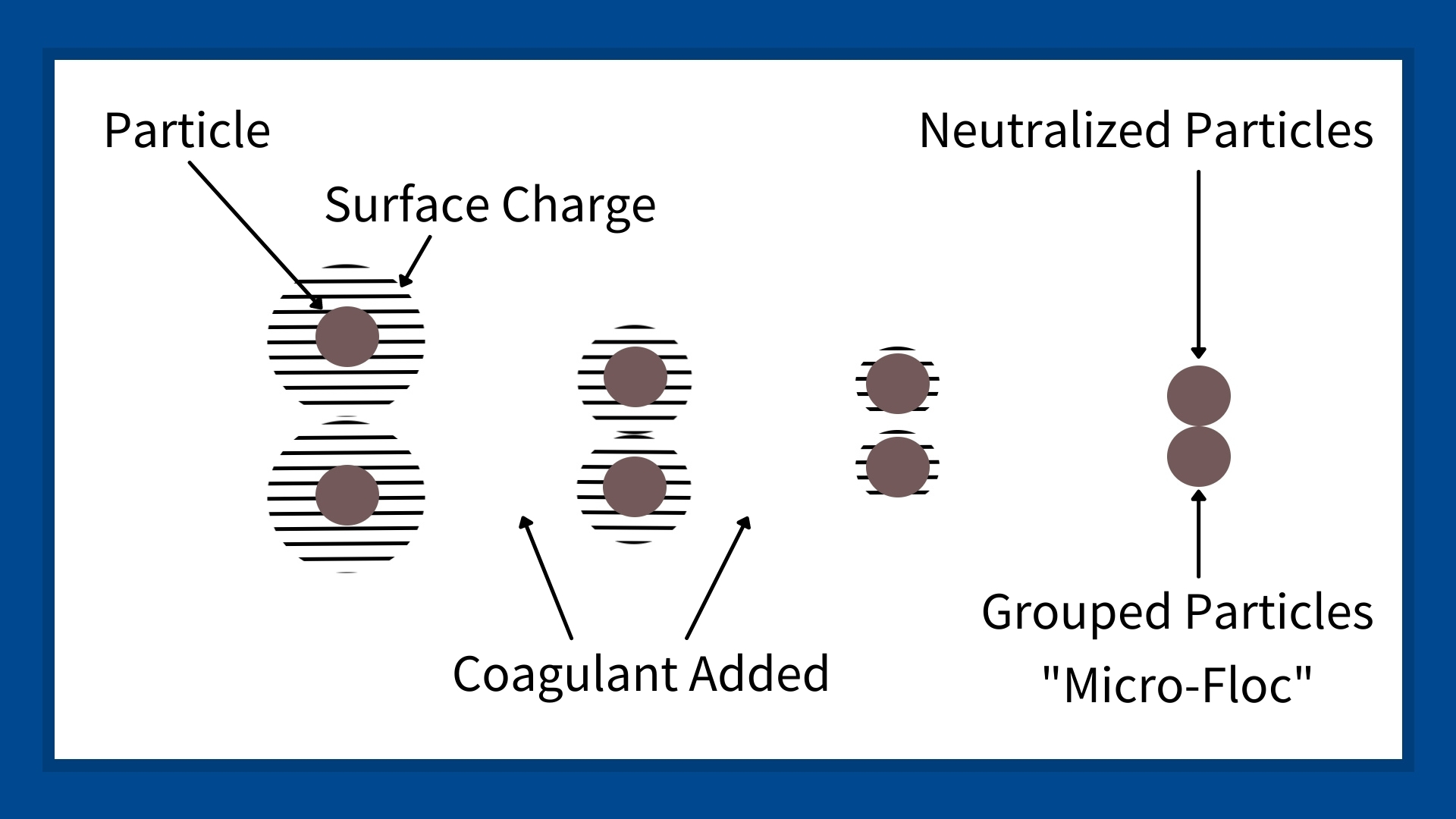
Most industrial process water or wastewater includes sediment and particles with a negative electric charge. The negative charge around each particle keeps them from coming together, creating colloidal dispersion. Negatively charged particles won’t come together and float in liquid suspension for hours, weeks, and even years. A simple example is muddy water created by aggregate producers who wash sand and gravel.
A coagulant added to muddy water creates a coagulation process that neutralizes the particles’ negative charge. Once neutralized, the particles can come together to form larger particles called micro-flocs or pin flocs.
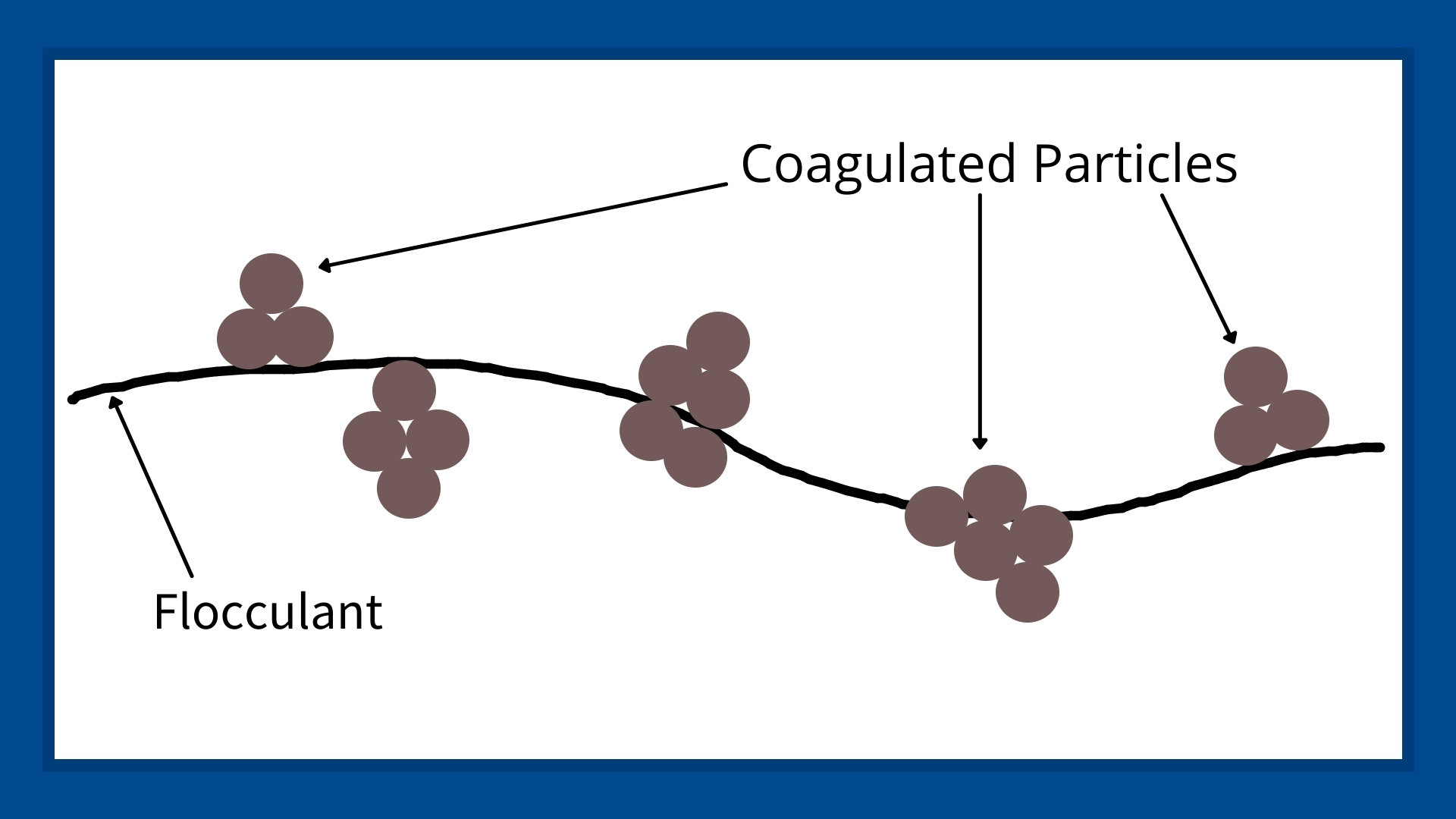
The next chemical reaction is called flocculation, which helps create even larger flocs or macro-flocs. In this step, a water-soluble polymer, or flocculant, helps bring together the coagulated particles to form longer and larger particle chains. As the flocculant chains become larger, they become heavier and even visible to the naked eye. The flocculants create a larger particle with a higher mass-to-drag ratio, speeding up the natural process of sedimentation.
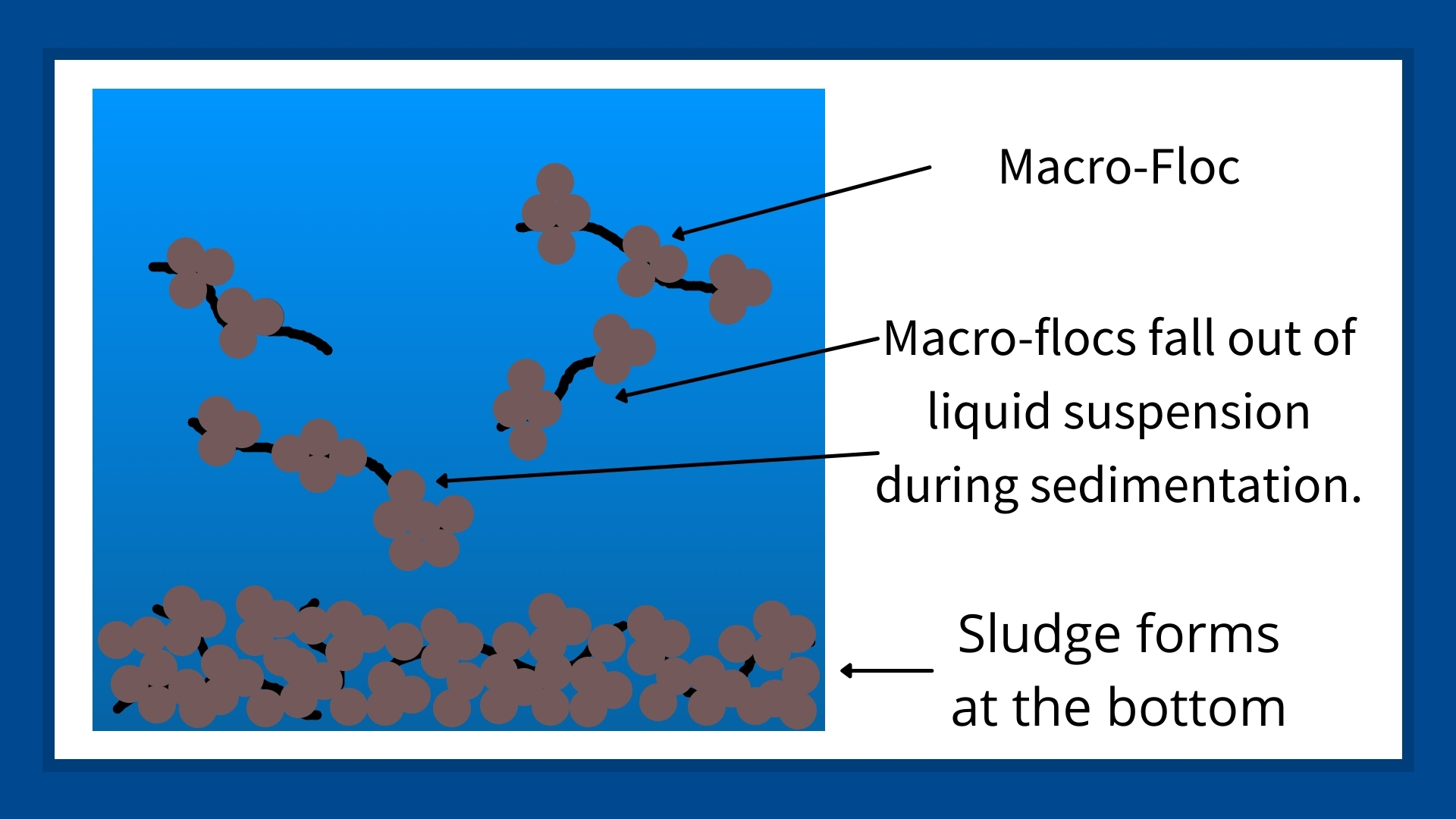
As the sedimentation process continues, a sludge forms at the bottom of whatever is holding the wastewater. In nature, we see this in the delta at a river’s mouth; in industry, this sludge accumulates in settling ponds, sludge ponds, clarifiers, or thickeners. Once the sludge has formed, it can be managed and disposed of through different methods.
It’s important to note that coagulants and flocculants aren’t only used to make sediment fall out of liquid suspension. Due to their ability to neutralize and agglomerate colloids, the chemicals also support dissolved air flotation (DAF) applications. In DAF applications, microbubbles pull the solids to the top of a tank, where a skimmer removes them.
As you can tell, polymers play an important role in wastewater treatment. Besides separating solids from liquids, they also help thicken sludge and dewater contaminated material for easier handling and disposal. Removing the water content from a waste sludge can change the waste properties from liquid to solid waste. This change has significant impacts on the disposal cost or tipping fee.
The industry standard determining whether the waste is solid or liquid is EPA Method 9095B or “the paint filter test.” In this procedure, a sample of the waste sludge is placed in a standard 60 mesh conical paint filter for 5 minutes. If no liquid passes through the filter, the waste is deemed solid and can be disposed of more cost-effectively than liquid waste.
In industry, we would consider sludge passing the paint filter test to be dewatered. Polymer flocculants expedite the dewatering processing by allowing water to leave the flocculated solids more freely.
This video shows the coagulation, flocculation, and sedimentation process in real-time.
Industries That Use Polymers to Treat Wastewater
Almost any industry that needs to remove solids from their wastewater stream can use polymers in their treatment process.
For instance, aggregate producers use water to wash the sand, gravel, or other aggregates they produce. The wash water picks up dirt, clay, and silt during the washing process. To reuse or safely discharge this wash water, the solids need to be separated from the water.
Many aggregate producers use settling ponds to hold the wastewater and allow sedimentation. As we noted, it can take a long time for sediment to fall out of liquid suspension. Aggregate producers must stop production while cleaning their ponds and waiting for clean water. These shutdowns cause producers to lose production hours and increase labor hours.
However, polymers can increase the rate of sedimentation for aggregate producers. By using a polymer dosing system, producers can inject the polymer solution directly into their settling ponds. The polymer dosing increases the sedimentation rate, provides clean water, and thickens the sludge at the pond’s bottom for easier management and disposal.
Many operations also use polymers to prepare wastewater before it’s mechanically treated in clarifiers, thickeners, filter presses, centrifuges, and other dewatering equipment.
The following video displays a polymer system coupled with a high compaction clarifier. This setup creates a thick and manageable mud discharge plus clean water for reuse.
It’s important to note that not all wastewater treatment polymers are the same. Specific chemistry is selected depending on the wastewater and the operation’s needs. Plus, as we said, dry and emulsion polymers require different dosing equipment. It can be tough to know what’s best for your application.
An excellent place to start is by speaking with an experienced application engineer. They will provide options that best fit your needs and use jar testing to determine the best polymer chemistry. The right type of polymer will help you meet regulations, save money, and increase your operation’s success.



